 |
|
You are not logged in! F.A.Q Log in Register |
 |

|
 |
dariusgriffin ...and 302 guests Last 5 registered Oplandisks nothingstar N_loop yipe foxtrotromeo Browse members... |
 |
Messages 2614103 Today 0 Topics 127542 |
|
|||||||||
| |||||||||
|
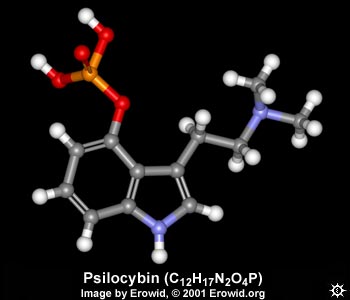
Hi. I purchased a simple molecular building set so i could recreate a psilocybin molecule. Can anyone tell me what those parallel bonds are? and what are the all grey bonds as opposed to the bonds that consist of 2 colours? In other words, what do the bonds consist of? I suppose in reality the atoms are just stuck together (without any 'sticks' inbetween so in the model they are just to keep things organized, right? But then there's the thing with the parallel sticks. Thing is, i only have these grey tubes for bonds, which means i cant recreate the connection from phosphorus to oxygen for example. I'll take pics of what i'm building tomorrow. thanks p.s: i wish i was an electron microscope times 1 million. |
|||||||||
| Attached picture | |||||||||
|
|||||||||
|
|
|||||||||
| |||||||||
|
the color of the bonds is just continuing the color of the athom, for aesthetical or enphatizing reasons. All bonds has the same meaning. The double bonds means... double bounds! its when two atoms have two ligations between then. So you must stick em with two bons - i hope they are flexible. electron microscopes cant see molecules even if its 1 million times because they are smaler than light wave lengh. |
|||||||||
|
|
|||||||||
| |||||||||
|
if electron microscopes cant see molecules, why and how can scientists alter them or synthesize certain chemicals? :( thanks for the info on the double bonds, i have some extra flexible tubes |
|||||||||
|
|
|||||||||
| |||||||||
|
thats why science rocks. often you dont need to see things to know how they are. you can measure it by experiments. |
|||||||||
|
|
|||||||||
| |||||||||
|
great. i have 6 and i need 8. this whole thing was an extremely bad idea. its horribly complex, and not as exciting as i thought it would be. |
|||||||||
|
|
|||||||||
| |||||||||
|
"if electron microscopes cant see molecules, why and how can scientists alter them or synthesize certain chemicals? :( " predictions. they predict that something will happen (this atom will stick to that atom) in case A (you mix this and that). Then they test it and see. |
|||||||||
|
|
|||||||||
| |||||||||
|
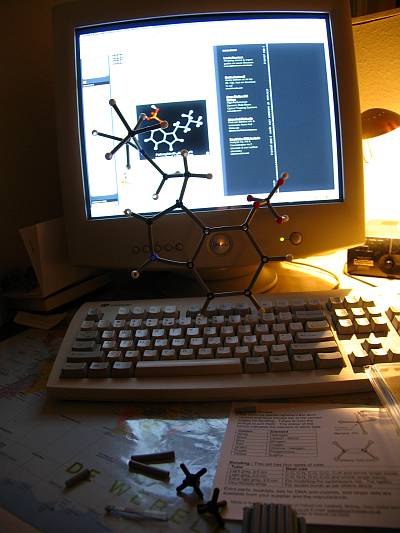
stefano, what are/were you studying? |
|||||||||
| Attached picture | |||||||||
|
|||||||||
|
|
|||||||||
| |||||||||
|
im at highschool. the model looks great, what will you do with it? |
|||||||||
|
|
|||||||||
| |||||||||
|
stefano_azevedo, J198 is a cunt who will just end up making Clogs, so he won't be doing anything with it. |
|||||||||
|
|
|||||||||
| |||||||||
|
normally in chemical structure kits all the bonds are the same colour. bonds are formed by sharing electrons. Electrons are arranged in quite a complicated way around the outside of an atom, and there are certain arrangements that are more stable than others; for example helium. Atoms bond to others in a way that makes them more stable. This is why helium doesnt appear in many molecules, but carbon does. The arrangement of electrons in carbon is such that it is most stable with 4 other electrons added to it - so it normally forms 4 bonds. I might try to make this molecule with my kit... |
|||||||||
|
|
|||||||||
| |||||||||
|
dont take that "fuck up" so seriously, it was not personal |
|||||||||
|
|
|||||||||
| |||||||||
|
I take nothing, nothing personally. A message board is very different than being face to face, when it comes to your manner - On a message board, I'll just treat the next person with same respect as they show me. That's fair, isn't it? |
|||||||||
|
|
|||||||||
| |||||||||
|
oh and the structures are known mostly from spectroscopy, there are several types, and its quite tricky. One example is to break up the molecule by firing electrons at it, and then it is possible to measure the fragments you get by their mass. considering we know the masses of any given atom, you can look at all the different fragment masses and try to piece together what they came from. For example if you see a fragment with a mass of 15 you can tell there is a methyl group (CH3) like those two on the right side of the psylocybin diagram, because C=12 and H=1. Then if you see also a 29, then perhaps that methyl is stuck on to a CH2 in the whole molecule... and so on. You need this together with other techniques which tell you different things... I spent too many hours doing this to not post in this topic |
|||||||||
|
|
|||||||||
| |||||||||
|
Bonding theory is incomplete. Electron distribution as predicted by quantum chemistry does not adequately explain inter-molecular bonding, especially the "double" bond. Quantum chemistry would not have the electrons between the atoms sharing the bond, but rather to either side. Drawing a pair of double lines (or placing a piece of plastic) between the atoms is a "fudge". |
|||||||||
|
|
|||||||||
| |||||||||
|
i dont believe there is a better explanation available, all of chemistry is a 'fudge' |
|||||||||
|
|
|||||||||
| |||||||||
|
chemistry is mostly a complete theory. |
|||||||||
|
|
|||||||||
| |||||||||
|
i rather read star signs wich i belive in |
|||||||||
|
|
|||||||||
| |||||||||
|
i hate all this quantum shit. in geology (where we do what the chemists i know call second-rate chemistry) we just treat atoms as the old billiard balls. the size of ions and the shape of the molecules correctly predicts the structure of minerals, so its good enough for us to avoid all the quantum stuff. it just seems to overcomplicate things. (obviously this is not the case in physics) |
|||||||||
|
|
|||||||||
| |||||||||
|
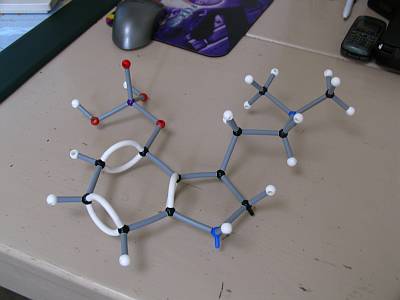
you guys rule. thanks a lot for trying to explain all this shit. here's the model as accurate as i can build it with this set, with just 2 extra flexible tubes (1 double bond) missing in the pentagon shape. Shame about the protruding nitrogen prongs too.. And i'm not yet sure whether the bond lenhth from Phosphorous should be short or long. The box reads: 3.5cm best used for: c-c, c-n, c-o, c=c, c=n and similar length bonds (wtf am i supposed to know those) 2.0cm best used for: c-h, n-h, o-h, c=o and similar length bonds. So you see there's no P to be seen. How can i find out? Dannn, are you building yet? |
|||||||||
|
|
|||||||||
| |||||||||
|
um, here: |
|||||||||
| Attached picture | |||||||||
|
|||||||||
|
|
|||||||||
| |||||||||
|
atoms are bond together in singular, double or triple legams in order to saturate, i think the color stands for a different element not sure, i'm a chemical engineer not a chemist |
|||||||||
|
|
|||||||||
| |||||||||
|
no, i went looking for my kit but I think i've given it to someone else at the moment. its normal for nitrogen to have 3 bonds so you i dont know why there are spare prongs... I feel very rusty on this now that I think about it, i never really used these kits very strictly, more just for a visual aid because thinking in 3D is too hard sometimes. I suppose if it says c-o bonds are long then so are p-o bonds. also on the phosphate group the p-o bond that doesnt have a hydrogen on the end (the one pointing up in your photo) is actually a double bond so you need even more bendy bonds. you cant really see it in the erowid picture |
|||||||||
|
|
|||||||||
| |||||||||
|
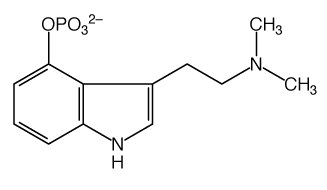
well spotted. looking at this confuses me even more though: |
|||||||||
| Attached picture | |||||||||
|
|||||||||
|
|
|||||||||
| |||||||||
|
a convention is to not write C and just put a bend in the line instead. and also C-H bonds are left off... its really for simplicity, because C always has 4 bonds and H always fills up any of those bonds that arent bonded to anything else. helps you see where the interesting parts of the molecule are. often nothing would be written where those CH3s are either |
|||||||||
|
|
|||||||||
| |||||||||
|
i remember building a TNT molecule once. wasnt too hard - just a benzene ring with some extra shit round it: CH3 C6 H2 (NO2)3 |
|||||||||
|
|
|||||||||
| |||||||||
|
i just found a cool toy on erowid but it requires you to install a plugin for which you have to register (free): http://www.erowid.org/plants/mushrooms/images/archive/psilo cybin__.mol it lets your rotate a 3d psilo molecule and you can modify some parameters. another problem with my model is that the the phosporus atom has only 4 prongs which are all in use. i'd need 2 more in order to connect a double bond :s kitty gnawing on pure psilocybin: |
|||||||||
| Attached picture | |||||||||

|
|||||||||
|
|
|||||||||
| |||||||||
|
oh yes... P shares 5 electrons |
|||||||||
|
|
|||||||||
|
Messageboard index
|
|||||||||

